货号:MD01
线粒体自噬检测试剂盒
Mitophagy Detection Kit
储存条件:0-5度保存
运输条件:室温
特点:
只需添加小分子量荧光试剂即可轻松检测线粒体
可以使用荧光显微镜进行活细胞成像
可以与附着的溶酶体染色剂同时染色
选择规格:1set
凑单关联产品TOP5
NO.1. Cell Counting Kit-8 细胞增殖毒性检测
NO.2. FerroOrange 细胞亚铁离子检测
NO.3. Cellular Senescence Detection Kit 细胞衰老检测
NO.4. Annexin V, FITC Apoptosis Detection Kit 细胞凋亡检测
NO.5. DAPGreen – Autophagy Detection 细胞自噬检测
试剂盒内含
| 1 set | .Mtphagy Dye . Lyso Dye |
×1管 ×1管 |

产品概述
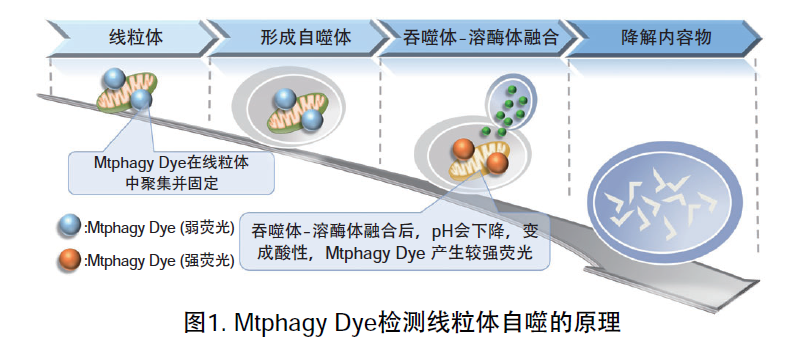
线粒体 (Mitochondria) 是细胞中重要的细胞器之一,可以为细胞活力提供能量 。近年有报道去极化线粒体的积累引起的阿尔茨海默病 (Alzheimer’s Disease) 与帕金森病(Parkinson’s Disease),可能与线粒体自噬有关。线粒体自噬是一种清除机制,可以通过自噬,将氧化应激、DNA损伤因素导致功能失调的线粒体隔离包裹成自噬体(Autophagosome),再与溶酶体 (Lysosome) 融合后降解。本试剂盒内含Mtphagy Dye (用于检测线粒体自噬) 和Lyso Dye (溶酶体染料)。Mtphagy Dye通过化学结合,固定在细胞内的线粒体上,会发出较弱的荧光。当线粒体发生自噬,损伤的线粒体会与溶酶体融合,pH会下降,变成酸性,此时Mtphagy Dye会产生较强的荧光。如想直观观察Mtphagy Dye标记的线粒体和溶酶体的结合,可联合应用试剂盒中的Lyso Dye (标记溶酶体) 进行双染。
特点:
1)只需添加小分子量荧光试剂即可轻松检测线粒体
2)可以使用荧光显微镜进行活细胞成像
3)可以与附着的溶酶体染色剂同时染色
原理
记载了本产品的检测原理和实验例的论文请看MD01论文实验例中第四篇:Live Cell Imaging of Mitochondrial Autophagy with a Novel Fluorescent Small Molecule
实验例
1.用羰基氰化物间氯苯腙 (CCCP,一种线粒体解偶联剂) 诱导Parkin表达的HeLa细胞线粒体自噬,并通过荧光显微镜进行检测。另外,通过与线粒体染色试剂(MitoBright Deep Red:MT08)一同染色,能够区分出已发生自噬的的线粒体(白色)和未发生自噬的线粒体(紫色)(照片:右侧)。

波长:
Mtphagy Dye:561 nm (Ex)、650 LP nm (Em)
Lyso Dye:488 nm (Ex)、502-554 nm (Em)
MitoBright Deep Red:640 nm (Ex)、656-700 nm (Em)
2.荧光显微镜观察
HeLa细胞用CCCP处理,并与线粒体检测试剂(Mtphagy Dye)和线粒体染色试剂(MitoBright LT Green)共同染色,并经过一段时间(6小时)后进行检测。
<检测条件>
设备:LSM-700 Laser scanning confocal microscope (LSCM)
(Carl Zeiss, Oberkochen, Germany)
激发波长:
MitoBright LT Green 488 nm
Mtphagy Dye 555 nm
物镜:63x
拍摄时间:6小时
拍摄间隔:15秒
3.自噬诱导和线粒体膜电位变化关系的检测
用羰基氰化物间氯苯腙(CCCP,一种线粒体解偶联剂)诱导Parkin表达的HeLa细胞线粒体自噬,并使用线粒体自噬检测试剂盒(Mitophagy Detection Kit:MD01)和线粒体膜电位检测试剂盒(JC-1 MitoMP Detection Kit:MT09)观察荧光结果。
结果证实在未经CCCP处理的细胞中几乎未检测到线粒体自噬的发生,并且线粒体膜电位正常维持。 另一方面,在添加了CCCP的细胞中,证实了线粒体膜电位的降低(JC-1的红色荧光降低)和线粒体自噬的发生(Mtphagy染料的荧光增强)。
<实验条件>
■将Parkin质粒导入HeLa细胞
使用HilyMax(货号:H357)将Parkin质粒引入HeLa细胞中(Parkin质粒/HilyMax试剂:0.1 μg/0.2 μl)
过夜培养后进行检测。
■线粒体自噬检测
向表达Parkin的HeLa细胞中添加0.1 μmol/l Mtphagy工作溶液,并在37°C下孵育30分钟。然后将细胞用HBSS洗涤,加入10 μg/ml CCCP/MEM溶液,并在37℃下孵育2小时。在荧光显微镜下观察细胞。
■线粒体膜电位检测
将10 μg/ml的CCCP/MEM溶液添加至表达Parkin的HeLa细胞中,并在37℃下孵育1.5小时。加入4 μmol/l的JC-1工作液使其终浓度达到2 μmol/l,并在37℃下孵育30分钟。孵育后,将细胞用HBSS洗涤,加入成像缓冲液,并在荧光显微镜下观察细胞。

<检测条件>
■线粒体自噬检测
Ex:561 nm,Em:570-700 nm
■线粒体膜电位检测
绿色Ex:488 nm,Em:500-550 nm
红色Ex:561 nm,Em:560-610 nm
荧光特性

参考文献
| 序号 | 检测对象 | 使用仪器 | 文献 |
| 1) | 细胞(HeLa) | 流式细胞仪 | J. Koniga, C. Otta, M. Hugoa, T. Junga, A. L. Bulteaub, T. Grunea and A. Hohna, “Mitochondrial contribution to lipofuscin formation”, Redox Biology, 2017, 11, 673. |
| 2) | 细胞(KB) | 荧光显微镜 | K. Kameyama, “Induction of mitophagy-mediated antitumor activity with folate-appended methyl-β-cyclodextrin”, International Journal of Nanomedicine, 2017, 12, 3433. |
| 3) | 细胞(SH-SY5Y, 初代皮质神经细胞) | 荧光显微镜 | E. F. Fang, T. B. Waltz, H. Kassahun, Q. Lu, J. S. Kerr, M. Morevati, E. M. Fivenson, B. N. Wollman, K. Marosi, M. A. Wilson, W. B. Iser, D. M. Eckley, Y. Zhang, E. Lehrmann, I. G. Goldberg, M. S. Knudsen, M. P. Mattson, H. Nilsen, V. A. Bohr and K. G. Becker, “Tomatidine enhances lifespan and healthspan in C. elegans through mitophagy induction via the SKN-1/Nrf2 pathway”, Scientific Reports, 2017, 7, (46208), DOI: 10.1038/srep46208. |
| 4) | 细胞(HeLa、Parkin表达HeLa) | 荧光显微镜 | H. Iwashita, S. Torii, N. Nagahora, M. Ishiyama, K. Shioji, K. Sasamoto, S. Shimizu and K. Okuma, “Live Cell Imaging of Mitochondrial Autophagy with a Novel Fluorescent Small Molecule”, ACS Chem. Biol., 2017, 12, (10), 2546. |
| 5) | 细胞(Cardiomyocytes) | 流式细胞仪 | Y. Feng, NB. Madungwe, CV. da Cruz Junho and JC. Bopassa, “Activation of G protein-coupled oestrogen receptor 1 at the onset of reperfusion protects the myocardium against ischemia/reperfusion injury by reducing mitochondrial dysfunction and mitophagy.”, Br. J. Pharmacol., 2017, 174, (23), 4329. |
| 6) | 细胞(HCT116) | 荧光显微镜 | K. M. Elamin, K. Motoyama, T. Higashi, Y. Yamashita, A. Tokuda and H. Arima, “Dual targeting system by supramolecular complex of folate-conjugated methyl-β-cyclodextrin with adamantane-grafted hyaluronic acid for the treatment of colorectal cancer.”, Int. J. Biol. Macromol., 2018, doi: 10.1016/j.ijbiomac.2018.02.149. |
| 7) | 细胞(Parkin-HeLa) | 流式细胞仪 | N. Furuya, S. Kakuta, K. Sumiyoshi, M. Ando, R. Nonaka, A. Suzuki, S. Kazuno, S. Saiki and N. Hattori, “NDP52 interacts with mitochondrial RNA poly(A) polymerase to promote mitophagy.”, EMBO Rep. ., 2018, doi: 10.15252/embr.201846363. |
| 8) | 细胞(NKT) | 流式细胞仪 | L. Zhu, X. Xie, L. Zhang, H. Wang, Z. Jie, X. Zhou, J. Shi, S. Zhao, B. Zhang, X. Cheng and S. Sun, “TBK-binding protein 1 regulates IL-15-induced autophagy and NKT cell survival”, Nature Communications., 2018, 9, (1), doi:10.1038/s41467-018-05097-5. |
| 9) | 细胞(HeLa) | 流式细胞仪 | K. Araki, K. Kawauchi, W. Sugimoto, D. Tsuda, H. Oda, R. Yoshida and K. Ohtani, “Mitochondrial protein E2F3d, a distinctive E2F3 product, mediates hypoxia-induced mitophagy in cancer cells”, Commun Biol., 2019, DOI: 10.1038/s42003-018-0246-9. |
| 10) | 细胞(Bovine Sertoli) | 荧光显微镜 | E. Adegoke, S. Adeniran, Y. Zeng, X. Wang, H. Wang, C. Wang, H. Zhang, P. Zheng and G. Zhang , “Pharmacological inhibition of TLR4/NF-κB with TLR4-IN-C34 attenuated microcystin-leucine arginine toxicity in bovine Sertoli cells.”, J Appl Toxicol., 2019,doi: 10.1002/jat.3771. |
| 11) | 组织(小鼠) | 荧光显微镜 | E. F. Fang, Y. Hou, K. Palikaras, B. A. Adriaanse, J. S. Kerr, B. Yang, S. Lautrup, M. M. Hasan-Olive, D. Caponio, X. Dan, P. Rocktaschel, D. L. Croteau, M. Akbari, N. H. Greig, T. Fladby, H. Nilsen, M. Z. Cader, M. P. Mattson, N. Tavernarakis and V. A. Bohr, “Mitophagy inhibits amyloid-β and tau pathology and reverses cognitive deficits in models of Alzheimer’s disease.”, Nat. Neurosci. ., 2019,DOI:10.1038/s41593-018-0332-9. |
| 12) | 细胞(HepG2) | 荧光显微镜 | Iwasawa, T. Shinomiya, N. Ota, N. Shibata, K. Nakata, I. Shiina, and Y. Nagahara , “Novel Ridaifen-B Structure Analog Induces Apoptosis and Autophagy Depending on Pyrrolidine Side Chain”, Biological and Pharmaceutical Bulletin., 2019, 42, (3), 401-410, doi: 10.1248/bpb.b18-00643. |
| 13) | 细胞(U2OS) | 荧光显微镜 | T. Namba, “BAP31 regulates mitochondrial function via interaction with Tom40 within ER-mitochondria contact sites “, Sci Adv., 2019, 5, (6), 1386. |
| 14) | 细胞(INS-1) | 荧光显微镜 | A. Inamura, S. M. Hirayama, and K. Sakurai, Loss of Mitochondrial DNA by Gemcitabine Triggers Mitophagy and Cell Death’, Biol. Pharm. Bull.., 2019, 42, 1977. |
| 15) | 细胞(HRCEpiC, HRPTEpic) | 流式细胞仪 | Y. Zhao and M. Sun, Metformin rescues Parkin protein expression and mitophagy in high glucose-challenged human renal epithelial cells by inhibiting NF-κB via PP2A activation., Life Sci.., 2020, DOI:10.1016/j.lfs.2020.117382. |
| 16) | 细胞(RAES) | 荧光显微镜 | N. Liu, J. Wu, L. Zhang, Z. Gao, Y. Sun, M. Yu, Y. Zhao, S. Dong, F. Lu and W. Zhang , “Hydrogen Sulphide modulating mitochondrial morphology to promote mitophagy in endothelial cells under high‐glucose and high‐palmitate “, J. Cell. Mol. Med., 2017, 21, (12), 3190. |
| 17) | 细胞(BAECs) | 荧光显微镜 | N. Kajihara, D. Kukidome, K. Sada, H. Motoshima, N. Furukawa, T. Matsumura, T. Nishikawa and E. Araki, “Low glucose induces mitochondrial reactive oxygen species via fatty acid oxidation in bovine aortic endothelial cells”, J Diabetes Investig, 2017, 8, (6), 750. |
| 18) | 细胞(HT22) | 荧光显微镜 | M. Jin, H. Ni and L. Li, “Leptin Maintained Zinc Homeostasis Against Glutamate-Induced Excitotoxicity by Preventing Mitophagy-Mediated Mitochondrial Activation in HT22 Hippocampal Neuronal Cells.”, Front Neurol, 2018, 9, (9), 332. |
| 19) | 细胞(BMDMs) | 流式细胞仪 | D. Bhatia, K. P. Chung, K. Nakahira, E. Patino, M. C. Rice, L. K. Torres, T. Muthukumar, A. M. Choi, O. M. Akchurin and M. E. Choi , “Mitophagy-dependent macrophage reprogramming protects against kidney fibrosis”, JCI Insight, 2019, 4, (23), e132826. |
| 20) | 细胞(U2OS) | 荧光显微镜 | J. Zheng, D. L. Croteau, V. A. Bohr and M. Akbari, “Diminished OPA1 expression and impaired mitochondrial morphology and homeostasis in Aprataxin-deficient cells. “, Nucleic Acids Res., 2019, 47, (8), 4086. |
| 21) | 细胞(HT22) | 荧光显微镜 | D. D. Wang, M. F. Jin, D. J. Zhao and H. Ni, “Reduction of Mitophagy-Related Oxidative Stress and Preservation of Mitochondria Function Using Melatonin Therapy in an HT22 Hippocampal Neuronal Cell Model of Glutamate-Induced Excitotoxicity”, Front Endocrinol (Lausanne), 2019, 10, 550. |
| 22) | 细胞(CD4+T-cells, HeLa) | 荧光显微镜 | A. Bektas, S. H. Schurman, M. G. Freire, A. Bektas, S. H. Schurman, M. G. Freire, C. A. Dunn, A. K. Singh, F. Macian, A. M. Cuervo, R. Sen and L. Ferrucci, “Age-associated changes in human CD4+ T cells point to mitochondrial dysfunction consequent to impaired autophagy.”, Aging (Albany NY)., 2019, 11, (21), 9234-9263. |
| 23) | 细胞(ALM) | 流式细胞仪 | T. Nechiporuk, S.E. Kurtz, O. Nikolova, T. Liu, C.L. Jones, A. D. Alessandro, R. C. Hill, A. Almeida, S. K. Joshi, M. Rosenberg, C. E. Tognon, A. V. Danilov, B. J. Druker, B. H. Chang, S. K McWeeney and J. W. Tyner, “The TP53 Apoptotic Network Is a Primary Mediator of Resistance to BCL2 Inhibition in AML Cells.”, Cancer Discov., 2019, 9, (7), 919. |
| 24) | 细胞(PK-15) | 荧光显微镜 | Y. Zhang, R. Sun, X. Li and W. Fang, “Porcine Circovirus 2 Induction of ROS Is Responsible for Mitophagy in PK-15 Cells via Activation of Drp1 Phosphorylation”, Viruses., 2020, 12, (3), 289. |
| 25) | 细胞(HCE) | 荧光显微镜 | Y. Huo, W. Chen, X. Zheng, J. Zhao, Q. Zhang, Y. Hou, Y. Cai, X. Lu and X. Jin , “The protective effect of EGF-activated ROS in human corneal epithelial cells by inducing mitochondrial autophagy via activation TRPM2.”, J. Cell. Physiol., 2020, DOI: 10.1002/jcp.29597. |
| 26) | 细胞(心肌细胞) | 荧光显微镜 | Y. Sun, F. Lu, X. Yu, B. Wang, J. Chen, F. Lu, S. Peng, X. Sun, M. Yu, H. Chen, Y. Wang, L. Zhang, N. Liu, H. Du, D. Zhao and W. Zhang, “Exogenous H2S Promoted USP8 Sulfhydration to Regulate Mitophagy in the Hearts of db/db Mice.”, Aging Dis., 2020, 11, (2), 269. |
| 27) | 细胞(HCFs) | 荧光显微镜 | R. Tanaka, M. Umemura, M. Narikawa, M. Hikichi, K. Osaw, T. Fujita, U. Yokoyama, T. Ishigami, K. Tamura and Y. Ishikawa, “Reactive fibrosis precedes doxorubicin-induced heart failure through sterile inflammation.”, ESC Heart Fail., 2020, 7, (2), 588. |
| 28) | 细胞(VSMCs) | 荧光显微镜 | C. Duan, L. Kuang, X. Xiang, J. Zhang, Y. Zhu, Y. Wu, Q. Yan, L. Liu and T. Li, “Drp1 regulates mitochondrial dysfunction and dysregulated metabolism in ischemic injury via Clec16a-, BAX-, and GSH- pathways “, Cell Death Dis., 2020, 11, 251. |
| 29) | 细胞(Bovine Sertoli) | 荧光显微镜 | E. O. Adegoke, W. Xue, N. S. Machebe, S. O. Adeniran, W. Hao, W. Chen, Z. Han, Z. Guixue and Z. Peng, “Sodium Selenite inhibits mitophagy, downregulation and mislocalization of blood-testis barrier proteins of bovine Sertoli cell exposed to microcystin-leucine arginine (MC-LR) via TLR4/NF-kB and mitochondrial signaling pathways blockage.”, Ecotoxicol. Environ. Saf., 2018, 116, 165. |
| 30) | 细胞(HeLa) | 荧光显微镜 | D. Takahashi, J. Moriyama, T. Nakamura, E. Miki, E. Takahashi, A. Sato, T. Akaike, K. I. Nakama and H. Arimoto, “AUTACs: Cargo-Specific Degraders Using Selective Autophagy. “, Mol. Cell, 2019, 76, (5), 797. |
| 31) | 细胞(primary hepatocyte) | 荧光显微镜 | H. Kim, J. H. Lee and J. W. Park, “IDH2 deficiency exacerbates acetaminophen hepatotoxicity in mice via mitochondrial dysfunction-induced apoptosis.”, Biochim Biophys Acta Mol Basis Dis, 2019, 1865, (9), 2333. |
| 32) | 细胞(C3H10T1/2s) | 荧光显微镜 | M. S. Rahman and Y. S. Kim, “PINK1-PRKN mitophagy suppression by Mangiferin promotes a brown-fat-phenotype via PKA-p38 MAPK signalling in murine C3H10T1/2”, Metabolism, 2020, 101, 154228. |
| 33) | 细胞(NHEKs) | 荧光显微镜 | S. Ikeoka and A. Kiso , “The Involvement of Mitophagy in the Prevention of UV-B-Induced Damage in Human Epidermal Keratinocytes “, J. Soc. Cosmet. Chem. Jpn., 2020, 54(3), 252. |

