Lumiprobe Cyanine dyes 蓝色染料
Cyanine dyes are molecules containing polymethine bridge between two nitrogen atoms with a delocalized charge:
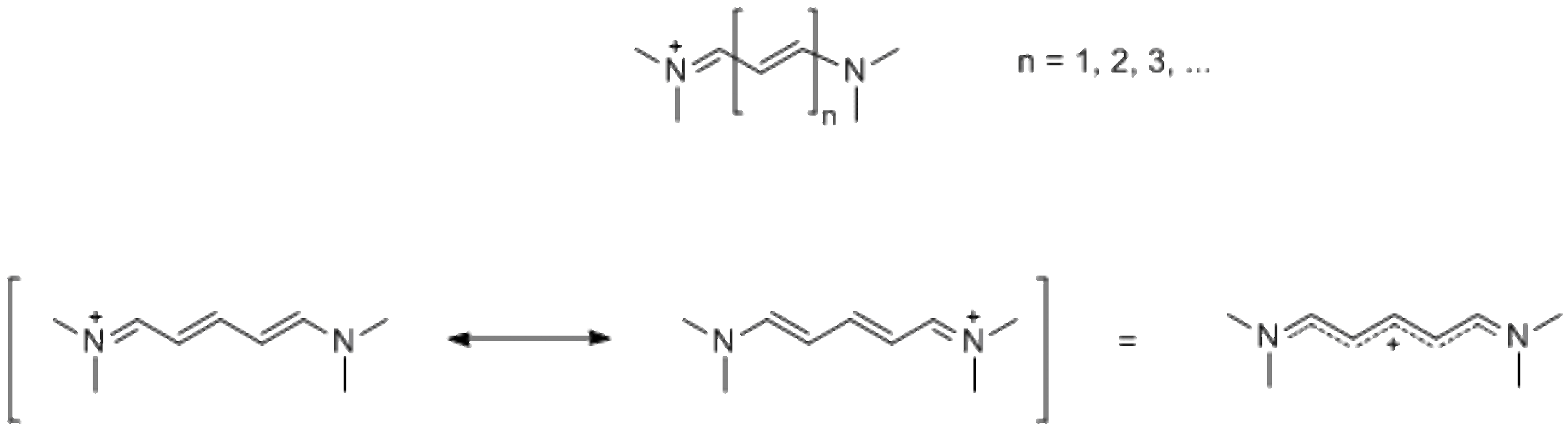
Due to their structure, cyanines have outstandingly high extinction coefficients often exceeding 100,000 Lmol-1cm-1. Different substituents allow to control properties of the chromophore, such as absorbance wavelength, photostability, and fluorescence. For example, absorbance and fluorescence wavelength can be controlled by a choice of polymethine bridge length: longer cyanines possess higher absorbance and emission wavelengths up to near infrared region.
A number of cyanine dyes have been used for life science applications. A series of thiazole and oxazole dyes have been used as DNA- and protein-binding dyes (like TOTO, YOYO, Stains All and others). But the most popular cyanine dyes for life science research were introduced by Alan Waggoner and colleagues of Carnegie Mellon University in early 1990s. The dyes were a modification of cyanine dye Indocyanine Green (ICG) which was used for angiography since 1970s, and they all contain two indolenine rings flanking polymethyne chain. The dyes were found to exhibit low non-specific binding to biomolecules, and have bright fluorescence owing to their huge extinction coefficients and good quantum yields. Once patented, these molecules are now in public domain after expiration of patents of CMU, and are available for purchase from Lumiprobe for research and commercial use as various reactive derivatives, such as NHS esters, maleimides, azides for Click chemistry, and other derivatives.
There are two varieties of cyanine dyes: non-sulfonated cyanines, and sulfonated cyanines. For many applications they are interchangeable, because their spectral properties are nearly identical. Both sulfonated and non-sulfonated dyes can be used for the labeling of biomolecules such as DNA and proteins. The difference between the dyes is their solubility: sulfo- dyes are water-soluble, and they do not use of organic co-solvent for the labeling in aqueous environment. They are less prone to aggregation in water. There are cases when one of the type of cyanines is desired (see Sulfonated vs non-sulfonated cyanines section below).
Non-sulfonated cyanines
Available non-sulfonated dyes incude Cy3, Cy3.5, Cy5, Cy5.5, Cy7, and Cy7.5. Cy® stands for ‘cyanine’, and the first digit identifies the number of carbon atoms between the indolenine groups. Cy2 which is an oxazole derivative rather than indolenin, is an exception from this rule. The suffix .5 is added for benzo-fused cyanines. Variation of the structures allows to change fluorescence properties of the molecules, and to cover most important part of visible and NIR spectrum with several fluorophores.
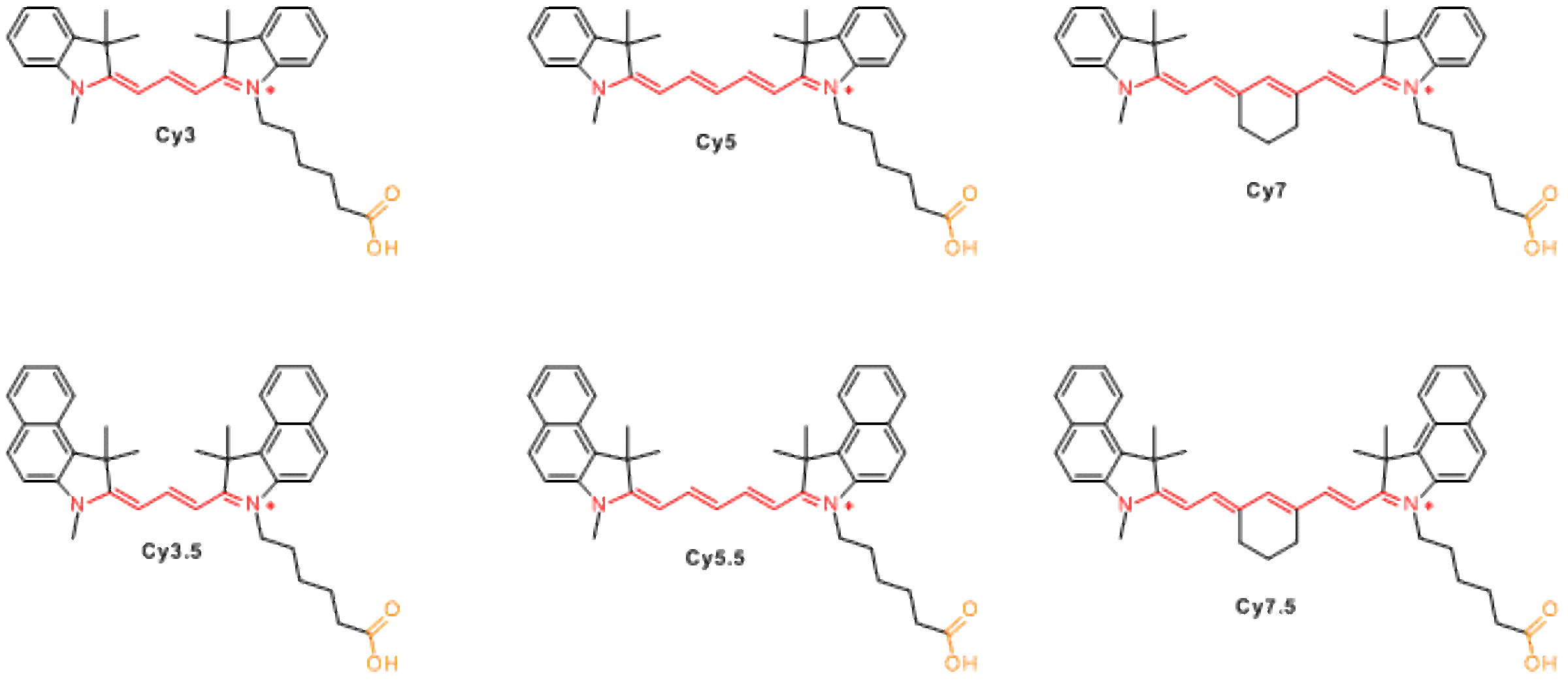
Most derivatives of non-sulfonated cyanines (except for hydrochlorides of hydrazides and amines) have low aqueous solubility. When these molecules are used for biomolecule labeling, use of organic co-solvent (5-20% of DMF or DMSO) is necessary for efficient reaction. Cyanine dye should be dissolved in organic solvent first, and added to a solution of biomolecule (protein, peptide, amino-labeled DNA) in appropriate aqueous buffer. When conjugation takes place efficiently, the dye reacts before it precipitates.
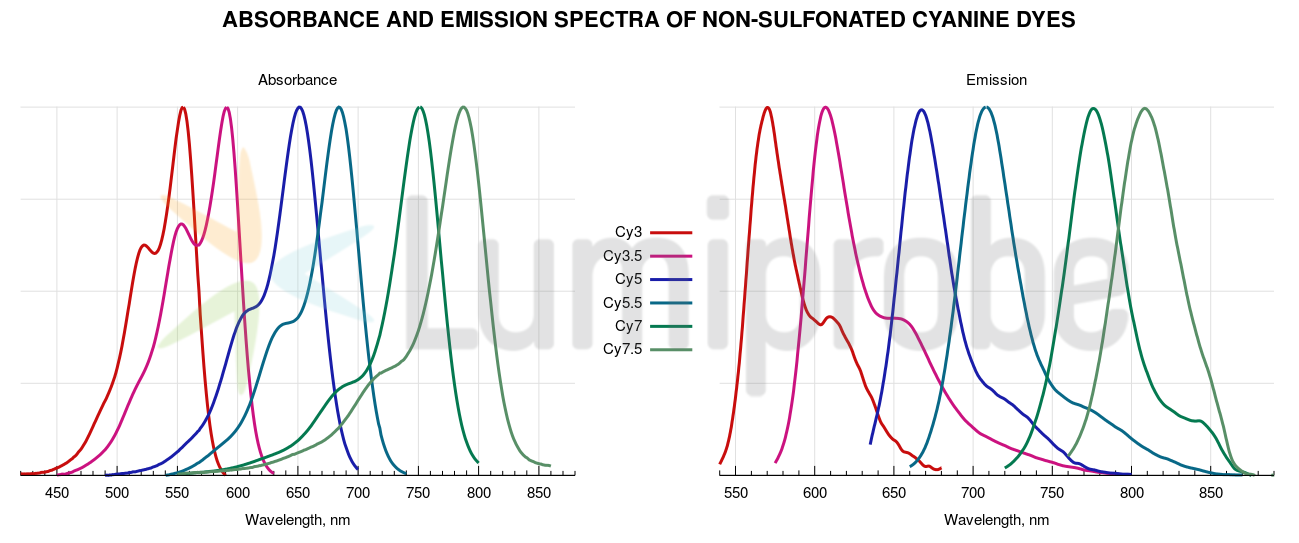
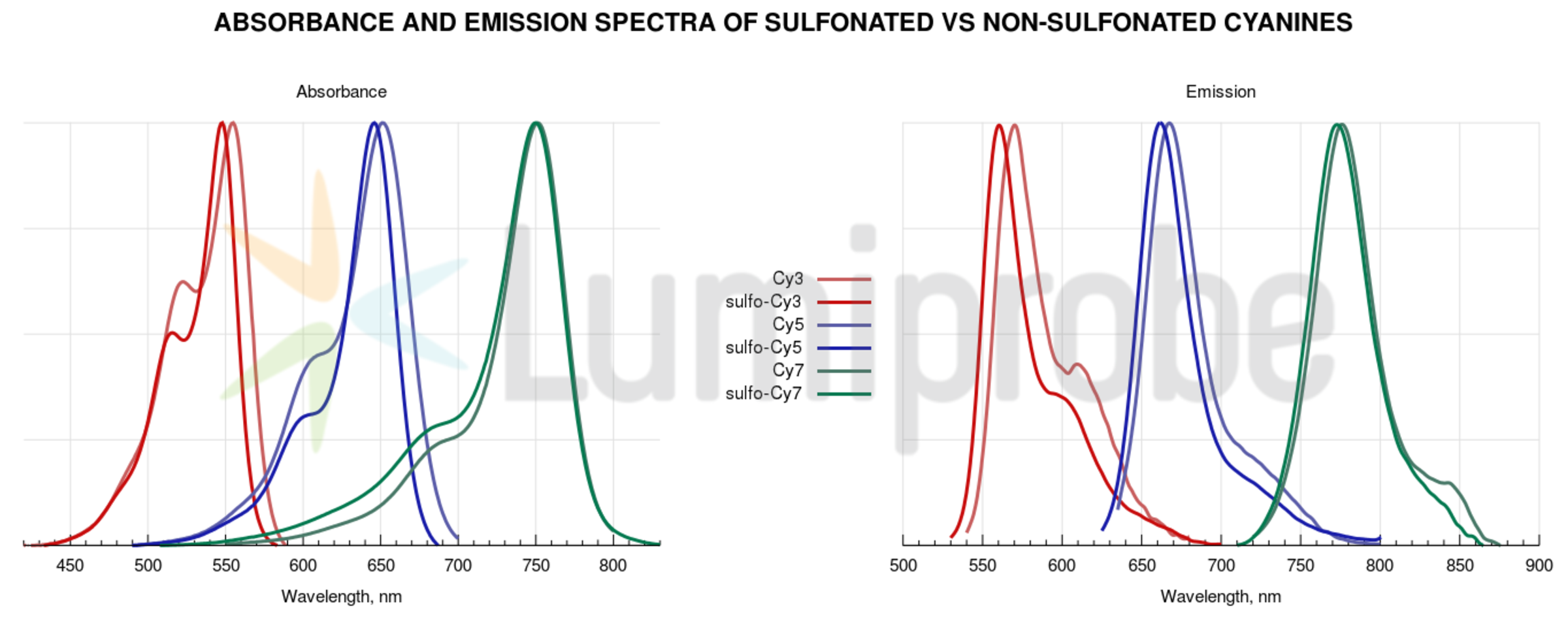
Fluorescent properties of non-sulfonated cyanines have little dependence on solvent and surrounding. Absorbance and fluorescence spectra of non-sulfonated cyanine dyes are plotted below.
Sulfonated cyanines
Sulfonated cyanines include additional sulfo-groups which facilitate dissolution of dye molecules in aqueous phase. Charged sulfonate groups decrease aggregation of dye molecules and heavily labeled conjugates.
Currently available sulfonated cyanines include sulfo-Cy3, sulfo-Cy5, and sulfo-Cy7.
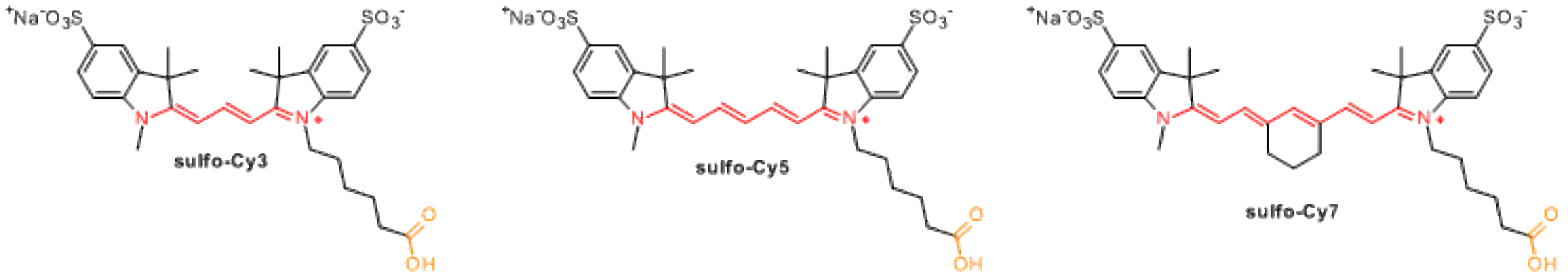
Sulfonated cyanines are highly water soluble. No organic co-solvent is needed to perform labeling with these reagents.
Sulfonated vs non-sulfonated cyanines
Sulfonated and non-sulfonated cyanines exhibit very similar fluorescent properties. However, there are a few differences in labeling protocols that should be noticed. Non-sulfonated cyanines must be dissolved in an organic co-solvent (DMF or DMSO) prior to use, and added to a solution of the target molecule in aqueous buffers. The recommended volume of co-solvent should be 10% for Cy3, Cy5, Cy7, and 15% for .5 counterparts. Sulfo-Cyanine reagents can be used in purely aqueous conditions. There is also a difference in purification: when dialysis against water or aqueous buffer is used for purification, sulfo-Cyanine must be used to achieve efficient removal of unreacted dye material. Reactions with both sulfo- and non-sulfo cyanines can be purified by gel filtration, chromatography (HPLC, FPLC, ion exchange), or electrophoresis.
Sulfonated and non-sulfonated cyanines are interchangeable for the labeling of many classes of targets including:
- soluble proteins, which are tolerant to addition of organic co-solvent
- antibodies (use 5-10% of DMSO/DMF)
- DNA and oligonucleotides
- peptides
- many small molecules
Conjugates produced with similar sulfo- and non-sulfonated reagents (for example, sulfo-Cy5 and Cy5) are very similar in their fluorescent properties, and can be used with various fluorescence instrumentation.
Sulfonated cyanines must be used for:
- sensitive proteins which are denatured by DMF or DMSO
- protein conjugation when purification is done by dialysis
- nanoparticles in aqueous solutions
- insoluble or hydrophobic proteins
Non-sulfonated cyanines must be used for:
- reactions in organic media (dichloromethane, acetonitrile)
The following cyanine products are available from Lumiprobe.
| Fluorophore |
Reactive form |
| sulfo-Cy3 |
NHS ester
maleimide
azide
alkyne
carboxylic acid |
| sulfo-Cy5 |
NHS ester
maleimide
azide
alkyne
carboxylic acid |
| sulfo-Cy7 |
NHS ester
azide
amine
carboxylic acid |
| Cy3 |
NHS ester
maleimide
azide
alkyne
hydrazide
amine
carboxylic acid |
| Cy3.5 |
NHS ester
azide
carboxylic acid |
| Cy5 |
NHS ester
maleimide
azide
alkyne
hydrazide
amine
carboxylic acid |
| Cy5.5 |
NHS ester
maleimide
azide
alkyne
hydrazide
amine
carboxylic acid |
| Cy7 |
NHS ester
maleimide
azide
alkyne
hydrazide
amine
carboxylic acid |
| Cy7.5 |
NHS ester
maleimide
azide
alkyne
hydrazide
amine
carboxylic acid |
Cy® is a trademark of GE Healthcare.
蓝色染料
蓝色染料是两个氮原子间含有聚甲基桥的分子,带去角电荷:

由于它们的结构,天鹅碱具有非常高的消光系数,常常超过100,000LMOL -1 共拍 -1 .不同的取代基可以控制色粒的性质,如吸收波长、光稳定性和荧光。例如,吸收率和荧光波长可以通过选择聚甲基桥长度来控制:较长的青素具有较高的吸收率和发射波长,直至近红外区域。
许多氰化染料已被用于生命科学应用。一系列的硫唑和硝唑染料已被用作DNA和蛋白质结合染料(如toto、yyo、染色等)。但是在生命科学研究中最流行的氰染料是由艾伦沃格纳和卡耐基梅隆大学的同事在上世纪90年代初介绍的。这些染料是对上世纪70年代以来用于血管造影的氰化染料因多氰化绿的一种修饰,它们都含有两个因多林环型聚甲基链。研究发现,染料与生物分子的非特异性结合较低,由于其消光系数大、量子产率好,具有明亮的荧光。一旦获得专利,这些分子在CMU专利到期后就会进入公共领域,可作为各种反应衍生物,如 NHS酯类 , 马来酰亚胺 , 氮化物 点击化学和其他衍生物。
蓝染料有两种:非磺化氰化染料和磺化氰化染料。对于许多应用来说,它们是可以互换的,因为它们的光谱特性几乎相同。磺化染料和非磺化染料均可用于标记DNA和蛋白质等生物分子。染料之间的区别在于它们的溶解性:硫染料是水溶性的,它们在水环境中不使用有机溶剂来标记。它们不太容易在水中聚集。有的情况下,一种类型的青素是理想的(参见 磺化与非磺化氰化 下一节)。
非磺酸盐
Available non-sulfonated dyes incude Cy3, Cy3.5, Cy5, Cy5.5, Cy7, and Cy7.5. Cy ® 表示”氰化物”,第一个数字表示因多林组之间碳原子的数量。是一种氧唑衍生物,而不是因多林,是这个规则的一个例外。后缀。5是对苯溶青素的添加。这种结构的变化能够改变分子的荧光性质,并能用几种荧光光覆盖可见光和NIR光谱的最重要部分。

大多数非磺化氰化类化合物(除了氢化氢氯化物和胺)具有较低的水溶性。当这些分子被用于生物分子标记时,使用有机溶剂(5-20%的DMF或DMSO)是有效反应的必要条件。氰化染料应首先在有机溶剂中溶解,并在适当的水缓冲液中加入生物分子(蛋白质、肽、氨基标记的DNA)。当共轭发生时,染料会在沉淀前发生反应。
非磺酸盐的荧光性能对溶剂和周围环境的依赖性较小。下面是非磺化氰化染料的吸收光谱和荧光光谱图。

磺酸盐
磺化氰化包括额外的磺基,以促进染料分子在水相中的溶解。电荷磺酸盐基团降低染料分子的聚集和重标记共轭物。
目前可获得的磺化氰化物包括磺化—-氰化3、磺化—-氰化5和磺化—-氰化7。

磺酸盐是高水溶性的。无需有机共溶剂对这些试剂进行标记。
磺化与非磺化氰化
磺化和非磺化氰化具有非常相似的荧光特性。然而,在标签协议方面有一些差异,值得注意。在使用之前,非磺酸盐必须溶解在有机共溶剂(DMF或DMSO)中,并添加到水缓冲剂中的目标分子溶液中。建议的共溶剂容量应是10%,对三、五、七和15%。5个对应方。磺胺-氰胺试剂可在纯水条件下使用。在净化方面也有区别:当对水进行透析或使用水缓冲来净化时,必须使用磺胺-氰胺来有效地去除未反应的染料物质。用凝胶过滤、色谱法(HPLC、FPLC、离子交换法)或电泳法对与硫和非硫氰烷的反应进行提纯。

磺化的和非磺化的氰化化合物可互换用于标记许多类别的目标,包括:
- 可溶性蛋白,对有机共溶剂的加入具有耐受性
- antibodies (use 5-10% of DMSO/DMF)
- DNA和寡核苷酸
- 多肽
- 许多小分子
与类似的磺化和非磺化试剂(例如,磺酸盐———————————————————–生产的共混物(例如,硫————
磺酸盐必须用于:
- 由DMF或DMSO变性的敏感蛋白
- 通过透析纯化蛋白质结合
- 水溶液中的纳米粒子
- 不溶性或疏水性蛋白
非磺胺必须用于: